Synthesis of Frameworks with Optimal Hydrogen Binding Enthalpy
Category
Synthesis
Laboratory
Lawrence Berkeley National Laboratory (LBNL)
Description
The realization of materials that strongly and reversibly adsorb hydrogen at ambient temperatures and moderate pressures could transform the transportation sector and vastly expand the adoption of fuel cells in other applications. It has been recognized that to maximize the deliverable H2 capacity under ambient working conditions, the enthalpy of H2 adsorption should fall within the optimal range of −15 to −25 kJ/mol.1,2 However, the majority of adsorbents studied for H2 storage exhibit binding enthalpies outside of this range, resulting in low hydrogen densities or prohibitively high regeneration energies. We recently reported the synthesis of the first metal–organic framework featuring exposed vanadium(II) sites, V2Cl2.8(btdd) (H2btdd, bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo[1,4]dioxin).3 These metal sites are capable of backbonding with weak π acids. Significantly, hydrogen adsorption data reveal that this material binds H2 at ambient temperatures with an enthalpy of −21 kJ/mol.4 This work demonstrates that engineering a high-density of Kubas-type vanadium(II)–dihydrogen complexation in frameworks is a suitable strategy to obtain ambient temperature adsorbents. The synthetic approach for V2Cl2.8(btdd) is being adapted to yield other vanadium(II) frameworks with an even higher density of open sites.
Status
Online and available for use in collaboration with HyMARC.
Figures
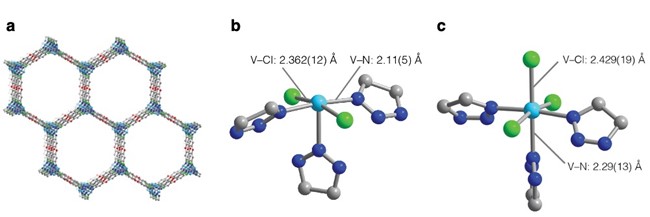
Figure 1. V2Cl2.8(btdd) structure as determined from powder X-ray diffraction, highlighting (a) the hexagon pores, (b) the five-coordinate vanadium(II) sites, and (c) the six-coordinate vanadium(III) sites. Cyan, green, blue, red, and grey spheres represent V, Cl, N, O, and C atoms, respectively. Terminal chloride ligands and H atoms have been omitted for clarity.
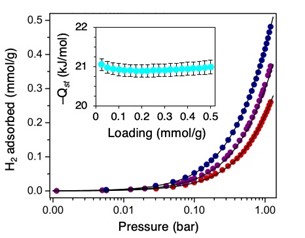
Figure 2. Low-pressure H2 isotherms at three temperatures are shown in a logarithmic plot. The fits (black lines) are shown, along with the isosteric heat of adsorption as a function of loading (inset). Blue, purple, and red correspond to 273, 286, and 298 K collections, respectively.
References
- K. Bhatia and A. L. Myers, Langmuir 22 (2006): 1688.
- S. Bae and R. Q. Snurr, Microporous Mesoporous Mater. 132 (2010): 300.
- E. Jaramillo, D. A. Reed, H. Z. H. Jiang, J. Oktawiec, M. W. Mara, A. C. Forse, D. J. Lussier, R. A. Murphy, M. Cunningham, V. Colombo, D. K. Shuh, J. A. Reimer and J. R. Long, Nat. Mater. 19 (2020): 517.
- E. Jaramillo, D. A. Reed, H. Z. H. Jiang, H. A. Evans, R. Chakraborty, H. Furukawa, C. M. Brown, M. Head-Gordon and J. R. Long, In preparation. (2020)
