Metal-Organic Framework (MOF) Monolith Synthesis
Category
Synthesis
Laboratory
Lawrence Berkeley National Laboratory (LBNL) and Sandia National Laboratory (SNL)
Description
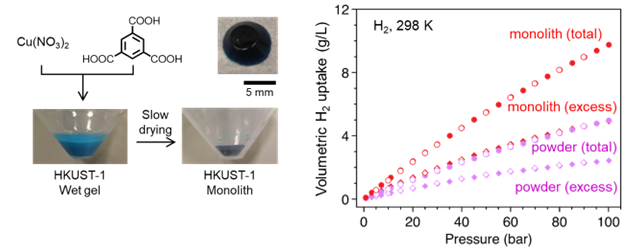
To minimize the losses in hydrogen storage capacity that result from packing adsorbent particles inside a tank, it is essential to compact the material in some fashion. Compaction reduces the interparticle void space and increases the bulk density of the powder; however, it often causes the collapse of framework structures. Recently a new strategy to increase the packing density of metal-organic frameworks (MOFs) is proposed, where a porous monolithic HKUST-1 was prepared by a sol-gel process to show volumetric methane uptake of the monolith is twice as much as that of powder.1 The HyMARC team is able to reproduce the monolith synthesis and demonstrated that total volumetric H2 uptake for the monolith is almost twice as high as that of hand-packed powder at 100 bar and 298 K. The HyMARC team is expanding this strategy to other framework materials, including Zr-based MOFs and M-MOF-74 (M = Ni, Co).
Status
Online and available for use in collaboration with HyMARC.
Figures
References
- 1. T. Tian, Z. Zeng, D. Vulpe, M. E. Casco, G. Divitini, P. A. Midgley, J. Silvestre-Albero, J.-C. Tan, P. Z. Moghadam and D. Fairen-Jimenez, Nat. Mater. 17 (2018): 174.
