Modeling of Interfaces in Hydrides
Category
Modeling/Simulation
Laboratory
Lawrence Livermore National Laboratory (LLNL)
Capability Experts
Tae Wook Heo ([email protected]), Brandon Wood ([email protected]), ShinYoung Kang ([email protected]), Keith Ray ([email protected]), Tadashi Ogitsu ([email protected])
Description
In many cases, kinetic limitations in metal hydrides are closely connected to the role of solid-solid interfaces and interface evolution. To investigate how interfaces affect the thermodynamics and kinetics of metal hydrides, HyMARC has developed multiscale combinatorial computations ranging from atomistic ab initio calculations to mesoscale phase field modeling and analytical isotherm modeling. Our multiphysics and multiscale modeling scheme is appropriate given structural, compositional, and chemical complexities of interfaces. Our interface modeling capabilities involve:
- Prediction of interface structures and energetics
- Microstructure-dependent phase evolution during (de)hydrogenation
- Interface chemistry and transport.
Besides solid-solid interfaces, interfaces with composite materials and confining media raise additional significance by extrinsic contamination and oxidation effects as well as intrinsic structural, compositional, and chemical complexities.
Figures
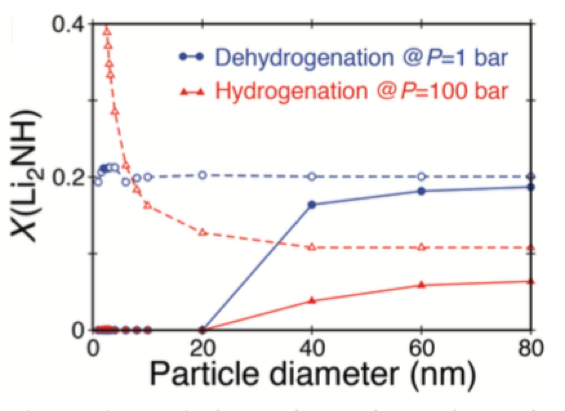
Predicted equilibrium mole fraction X of an intermediate phase, Li2NH, following hydrogenation at P = 100 bar (red solid line) and dehydrogenation at P = 1 bar (blue solid line) as a function of particle diameter. Dashed lines are the equivalent results when interfacial free energy contributions are neglected [1].
References
- B. C. Wood, V. Stavila, N. Poonyayant, T. W. Heo, K. G. Ray, L. E. Klebanoff, T. J. Udovic, J. R. I. Lee, N. Angboonpong, J. D. Sugar and P. Pakawatpanurut, “Nanointerface-driven reversible hydrogen storage in the nanoconfined Li-N-H system,” Adv. Mater. Interfaces 4 (2017): 1600803.
- S. Kang, T. Ogitsu, S. A. Bonev, T. W. Heo, M. D. Allendorf, and B. C. Wood, “Understanding charge transfer at Mg/MgH2 interfaces for hydrogen storage,” ECS Trans. 77 (2017): 81.
