Nanostructured Metal Hydrides
Category
Synthesis
Description
Nanosizing and nanoscaffolding are important strategies for controlling the kinetics, equilibrium pressure, and reversibility of lightweight metal hydrides.1 Reducing the size of metal hydrides to the nanometer range enables fast kinetics for both hydrogen release and subsequent uptake. Nanoscaling can also alter desorption thermodynamics by eliminating intermediate reaction steps, whereas surfaces and interfaces can also alter reaction pathways relative to bulk and enable reversibility. HyMARC maintains extensive facilities for the design, synthesis, and characterization of nanostructured and nanoconfined metal hydrides and has a team of experts to solve important technical problems in this area of research. The HyMARC team is developing nanoscale metal hydrides that desorb H2 with very little energy input and can also be rapidly rehydrided during refueling at a hydrogen filling station. Existing capabilities for synthesizing nanostructured hydrides include attrition, mechanochemistry, surfactant-assisted synthesis, and pulsed-laser deposition. Methods are also available to incorporate metal hydrides in a variety of porous hosts, including metal-organic frameworks (MOFs), graphene aerogels, and templated carbons. For example, we have employed melt infiltration to prepare Ti-doped NaAlH4@MOF-74(Mg)2 (Figure 1), and Li3N has been introduced into both MOFs and nanoporous carbons through the use of lithium solvated in liquid ammonia (Figure 2).3,4 HyMARC investigations probing the altered thermodynamics and reaction pathways are focused on modeling these nanoscale systems and applying the information to design new hydrides.
Status
Theoretical, synthetic, and characterization capabilities on line and fully functional.
Figures
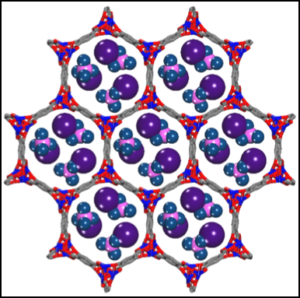
Figure 1. Schematic of Ti-doped NaAlH4 confined in the pores of MOF-74(Mg) with melt infiltration.

Figure 2. Zero-loss (left) and Li EFTEM (right) images of [email protected] porous carbon prepared with a solution of Li in liquid NH3.
References
- P. De Jongh, M. D. Allendorf, J. J. Vajo, C. Zlotea, "Nanoconfined light metal hydrides for reversible hydrogen storage, " MRS Bulletin 38 (2013): 488–494.
- V. Stavila, R. K. Bhakta, T. M. Alam, E. H. Majzoub, M. D. Allendorf, "Reversible Hydrogen Storage by NaAlH4 Confined within a Titanium-Functionalized MOF-74(Mg) Nanoreactor, " ACS Nano 6, no. 11 (2012): 9807–9817.
- V. Stavila, L. Klebanoff, "Nanostructured metal amides and nitrides for hydrogen storage, " US Patent Application 62/235,930, November 2015.
- B. C. Wood, V. Stavila, N. Poonyayant, T. W. Heo, K. Ray, L. E. Klebanoff, T. J. Udovic, J. R. I. Lee, N. Angboonpong, P. Pakawatpanurut, "Nanointerface-driven Reversible Hydrogen Storage in the Nanoconfined Li-N-H System, " Advanced Energy Materials, submitted.
